
April: SMH at Forefront of COVID-19 Research
Since the beginning of the COVID-19 pandemic, Sarasota Memorial has been among selected U.S. hospitals participating in multiple clinical research trials focused on preventing and treating the disease.
In April 2021, the hospital announced that it had a new experimental treatment in its arsenal to try to dampen the deadly inflammatory response in some patients hospitalized with severe COVID-19.
SMH was one of more than a dozen research sites across the nation participating in a trial of IC14, an anti-CD14 monoclonal antibody drug that researchers hope will reduce dangerous levels of inflammation in COVID-19 patients.
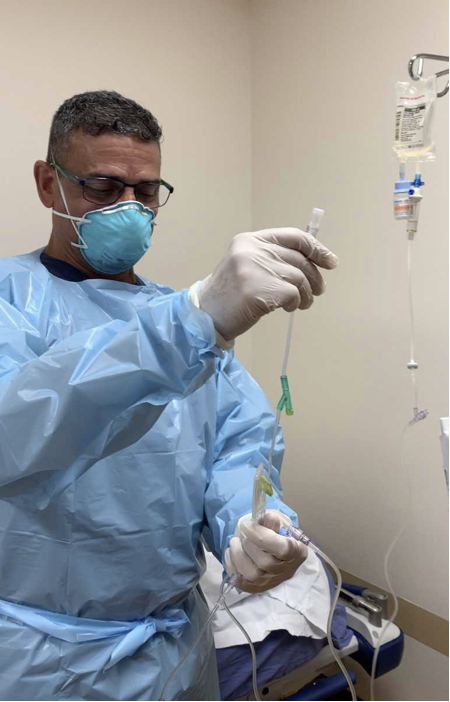
Also in the Spring of 2021, the drug maker Regeneron announced that a monoclonal antibody cocktail cut the risk of hospitalization and death when it was given to high-risk COVID-19 patients in a large clinical trial.
SMH was among 27 sites participating in the trial, which produced evidence that the treatment can aid recovery early in the course of the disease.
The Regeneron study found that patients who received the infused treatment within 10 days of developing symptoms or testing positive had a roughly 70% reduced risk of being hospitalized or dying compared with patients who were infused with a placebo. The trial also found that the treatment reduced the median recovery time from 14 days to 10.
When the number of COVID-19 cases escalated rapidly in Florida later in 2021, Sarasota Memorial provided the monoclonal antibody therapy to more than 1,000 eligible outpatients who had been recently diagnosed with a mild-to-moderate case of COVID-19.
The hospital still offers the outpatient therapy to COVID patients. For the latest information about the treatment from SMH experts, including its potential impact on the Omicron variant, click here.
